Hormesis
This article needs to be updated. The reason given is: seems relatively widely accepted concept now. (December 2023) |
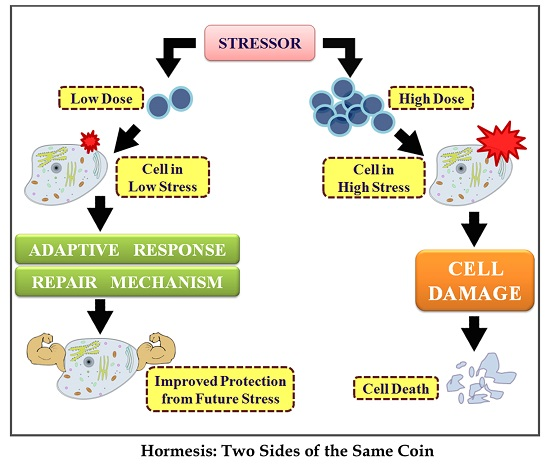
Hormesis is a two-phased dose-response relationship to an environmental agent whereby low-dose amounts have a beneficial effect and high-dose amounts are either inhibitory to function or toxic.[1][2][3][4] Within the hormetic zone, the biological response to low-dose amounts of some stressors is generally favorable. An example is the breathing of oxygen, which is required in low amounts (in air) via respiration in living animals, but can be toxic in high amounts, even in a managed clinical setting.[5]
In toxicology, hormesis is a dose-response phenomenon to xenobiotics or other stressors. In physiology and nutrition, hormesis has regions extending from low-dose deficiencies to homeostasis, and potential toxicity at high levels.[6] Physiological concentrations of an agent above or below homeostasis may adversely affect an organism, where the hormetic zone is a region of homeostasis of balanced nutrition.[7] In pharmacology, the hormetic zone is similar to the therapeutic window.
In the context of toxicology, the hormesis model of dose response is vigorously debated.[8] The biochemical mechanisms by which hormesis works (particularly in applied cases pertaining to behavior and toxins) remain under early laboratory research and are not well understood.[6]
Etymology
[edit]The term "hormesis" derives from Greek hórmēsis for "rapid motion, eagerness", itself from ancient Greek hormáein to excite.[4] The same Greek root provides the word hormone. The term "hormetics" is used for the study of hormesis.[6] The word hormesis was first reported in English in 1943.[4]
History
[edit]A form of hormesis famous in antiquity was Mithridatism, the practice whereby Mithridates VI of Pontus supposedly made himself immune to a variety of toxins by regular exposure to small doses. Mithridate and theriac, polypharmaceutical electuaries claiming descent from his formula and initially including flesh from poisonous animals, were consumed for centuries by emperors, kings, and queens as protection against poison and ill health. In the Renaissance, the Swiss doctor Paracelsus said, "All things are poison, and nothing is without poison; the dosage alone makes it so a thing is not a poison."
German pharmacologist Hugo Schulz first described such a phenomenon in 1888 following his own observations that the growth of yeast could be stimulated by small doses of poisons. This was coupled with the work of German physician Rudolph Arndt, who studied animals given low doses of drugs, eventually giving rise to the Arndt–Schulz rule.[8] Arndt's advocacy of homeopathy contributed to the rule's diminished credibility in the 1920s and 1930s.[8] The term "hormesis" was coined and used for the first time in a scientific paper by Chester M. Southam and J. Ehrlich in 1943 in the journal Phytopathology, volume 33, pp. 517–541.
In 2004, Edward Calabrese evaluated the concept of hormesis.[9][10] Over 600 substances show a U-shaped dose–response relationship; Calabrese and Baldwin wrote: "One percent (195 out of 20,285) of the published articles contained 668 dose-response relationships that met the entry criteria [of a U-shaped response indicative of hormesis]"[11]
Examples
[edit]Carbon monoxide
[edit]Carbon monoxide is produced in small quantities across phylogenetic kingdoms, where it has essential roles as a neurotransmitter (subcategorized as a gasotransmitter). The majority of endogenous carbon monoxide is produced by heme oxygenase; the loss of heme oxygenase and subsequent loss of carbon monoxide signaling has catastrophic implications for an organism.[12] In addition to physiological roles, small amounts of carbon monoxide can be inhaled or administered in the form of carbon monoxide-releasing molecules as a therapeutic agent.[13]
Regarding the hormetic curve graph:
- Deficiency zone: an absence of carbon monoxide signaling has toxic implications
- Hormetic zone / region of homeostasis: small amount of carbon monoxide has a positive effect:
- essential as a neurotransmitter
- beneficial as a pharmaceutical
- Toxicity zone: excessive exposure results in carbon monoxide poisoning[14]
Oxygen
[edit]Many organisms maintain a hormesis relationship with oxygen, which follows a hormetic curve similar to carbon monoxide:
- Deficiency zone: hypoxia / asphyxia
- Hormetic zone / region of homeostasis
- Toxicity zone: oxidative stress[5]
Physical exercise
[edit]Physical exercise intensity may exhibit a hormetic curve. Individuals with low levels of physical activity are at risk for some diseases; however, individuals engaged in moderate, regular exercise may experience less disease risk.[15]
Mitohormesis
[edit]The possible effect of small amounts of oxidative stress is under laboratory research.[16] Mitochondria are sometimes described as "cellular power plants" because they generate most of the cell's supply of adenosine triphosphate (ATP), a source of chemical energy. Reactive oxygen species (ROS) have been discarded as unwanted byproducts of oxidative phosphorylation in mitochondria by the proponents of the free-radical theory of aging promoted by Denham Harman. The free-radical theory states that compounds inactivating ROS would lead to a reduction of oxidative stress and thereby produce an increase in lifespan, although this theory holds only in basic research.[17] However, in over 19 clinical trials, "nutritional and genetic interventions to boost antioxidants have generally failed to increase life span."[18]
Whether this concept applies to humans remains to be shown, although a 2007 epidemiological study supports the possibility of mitohormesis, indicating that supplementation with beta-carotene, vitamin A or vitamin E may increase disease prevalence in humans.[19] More recent studies have reported that rapamycin exhibits hormesis, where low doses can enhance cellular longevity by partially inhibiting mTOR, unlike higher doses that are toxic due to complete inhibition. This partial inhibition of mTOR (by the hormetic effect of low-dose rapamycin) modulates mTOR–mitochondria cross-talk, thereby demonstrating mitohormesis; and consequently reducing oxidative damage, metabolic dysregulation, and mitochondrial dysfunction, thus slowing cellular aging.[2][3]
Alcohol
[edit]Alcohol is believed to be hormetic in preventing heart disease and stroke,[20] although the benefits of light drinking may have been exaggerated.[21][22] The gut microbiome of a typical healthy individual naturally ferments small amounts of ethanol, and in rare cases dysbiosis leads to auto-brewery syndrome, therefore whether benefits of alcohol are derived from the behavior of consuming alcoholic drinks or as a homeostasis factor in normal physiology via metabolites from commensal microbiota remains unclear.[23][24]
In 2012, researchers at UCLA found that tiny amounts (1 mM, or 0.005%) of ethanol doubled the lifespan of Caenorhabditis elegans, a roundworm frequently used in biological studies, that were starved of other nutrients. Higher doses of 0.4% provided no longevity benefit.[25] However, worms exposed to 0.005% did not develop normally (their development was arrested). The authors argue that the worms were using ethanol as an alternative energy source in the absence of other nutrition, or had initiated a stress response. They did not test the effect of ethanol on worms fed a normal diet.
Methylmercury
[edit]In 2010, a paper in the journal Environmental Toxicology & Chemistry showed that low doses of methylmercury, a potent neurotoxic pollutant, improved the hatching rate of mallard eggs.[26] The author of the study, Gary Heinz, who led the study for the U.S. Geological Survey at the Patuxent Wildlife Research Center in Beltsville, stated that other explanations are possible. For instance, the flock he studied might have harbored some low, subclinical infection and that mercury, well known to be antimicrobial, might have killed the infection that otherwise hurt reproduction in the untreated birds.[26]
Radiation
[edit]Ionizing radiation
[edit]Hormesis has been observed in a number of cases in humans and animals exposed to chronic low doses of ionizing radiation. A-bomb survivors who received high doses exhibited shortened lifespan and increased cancer mortality, but those who received low doses had lower cancer mortality than the Japanese average.[27][28]
In Taiwan, recycled radiocontaminated steel was inadvertently used in the construction of over 100 apartment buildings, causing the long-term exposure of 10,000 people. The average dose rate was 50 mSv/year and a subset of the population (1,000 people) received a total dose over 4,000 mSv over ten years. In the widely used linear no-threshold model used by regulatory bodies, the expected cancer deaths in this population would have been 302 with 70 caused by the extra ionizing radiation, with the remainder caused by natural background radiation. The observed cancer rate, though, was quite low at 7 cancer deaths when 232 would be predicted by the LNT model had they not been exposed to the radiation from the building materials. Ionizing radiation hormesis appears to be at work.[29]
Chemical and ionizing radiation combined
[edit]No experiment can be performed in perfect isolation. Thick lead shielding around a chemical dose experiment to rule out the effects of ionizing radiation is built and rigorously controlled for in the laboratory, and certainly not the field. Likewise the same applies for ionizing radiation studies. Ionizing radiation is released when an unstable particle releases radiation, creating two new substances and energy in the form of an electromagnetic wave. The resulting materials are then free to interact with any environmental elements, and the energy released can also be used as a catalyst in further ionizing radiation interactions.[30]
The resulting confusion in the low-dose exposure field (radiation and chemical) arise from lack of consideration of this concept as described by Mothersill and Seymory.[31]
Nucleotide excision repair
[edit]Veterans of the Gulf War (1991) who suffered from the persistent symptoms of Gulf War Illness (GWI) were likely exposed to stresses from toxic chemicals and/or radiation.[32] The DNA damaging (genotoxic) effects of such exposures can be, at least partially, overcome by the DNA nucleotide excision repair (NER) pathway. Lymphocytes from GWI veterans exhibited a significantly elevated level of NER repair.[32] It was suggested that this increased NER capability in exposed veterans was likely a hormetic response, that is, an induced protective response resulting from battlefield exposure.[32]
Applications
[edit]Effects in aging
[edit]One of the areas where the concept of hormesis has been explored extensively with respect to its applicability is aging.[33][34] Since the basic survival capacity of any biological system depends on its homeostatic ability, biogerontologists proposed that exposing cells and organisms to mild stress should result in the adaptive or hormetic response with various biological benefits. This idea has preliminary evidence showing that repetitive mild stress exposure may have anti-aging effects in laboratory models.[35][36] Some mild stresses used for such studies on the application of hormesis in aging research and interventions are heat shock, irradiation, prooxidants, hypergravity, and food restriction.[35][36][37] Such compounds that may modulate stress responses in cells have been termed "hormetins".[35]
Controversy
[edit]Hormesis suggests dangerous substances have benefits. Concerns exist that the concept has been leveraged by lobbyists to weaken environmental regulations of some well-known toxic substances in the US.[38]
Radiation controversy
[edit]The hypothesis of hormesis has generated the most controversy when applied to ionizing radiation. This hypothesis is called radiation hormesis. For policy-making purposes, the commonly accepted model of dose response in radiobiology is the linear no-threshold model (LNT), which assumes a strictly linear dependence between the risk of radiation-induced adverse health effects and radiation dose, implying that there is no safe dose of radiation for humans.
Nonetheless, many countries including the Czech Republic, Germany, Austria, Poland, and the United States have radon therapy centers whose whole primary operating principle is the assumption of radiation hormesis, or beneficial impact of small doses of radiation on human health. Countries such as Germany and Austria at the same time have imposed very strict antinuclear regulations, which have been described as radiophobic inconsistency.
The United States National Research Council (part of the National Academy of Sciences),[39] the National Council on Radiation Protection and Measurements (a body commissioned by the United States Congress)[40] and the United Nations Scientific Committee on the Effects of Ionizing Radiation all agree that radiation hormesis is not clearly shown, nor clearly the rule for radiation doses.
A United States–based National Council on Radiation Protection and Measurements stated in 2001 that evidence for radiation hormesis is insufficient and radiation protection authorities should continue to apply the LNT model for purposes of risk estimation.[40]
A 2005 report commissioned by the French National Academy concluded that evidence for hormesis occurring at low doses is sufficient and LNT should be reconsidered as the methodology used to estimate risks from low-level sources of radiation, such as deep geological repositories for nuclear waste.[41]
Policy consequences
[edit]Hormesis remains largely unknown to the public, requiring a policy change for a possible toxin to consider exposure risk of small doses.[42]
See also
[edit]- Calorie restriction
- Michael Ristow
- Petkau effect
- Radiation hormesis
- Stochastic resonance
- Mithridatism
- Antifragility
- Xenohormesis
References
[edit]- ^ a b Bhakta-Guha, Dipita; Efferth, Thomas (2015-12-16). "Hormesis: Decoding Two Sides of the Same Coin". Pharmaceuticals (Basel, Switzerland). 8 (4): 865–883. doi:10.3390/ph8040865. ISSN 1424-8247. PMC 4695814. PMID 26694419.
- ^ a b Mahalakshmi, R.; Priyanga, J.; Bhakta-Guha, Dipita; Guha, Gunjan (2022-01-01). "Hormetic effect of low doses of rapamycin triggers anti-aging cascades in WRL-68 cells by modulating an mTOR-mitochondria cross-talk". Molecular Biology Reports. 49 (1): 463–476. doi:10.1007/s11033-021-06898-6. ISSN 1573-4978. PMID 34739690.
- ^ a b Mahalakshmi, R.; Priyanga, J.; Bhakta-Guha, Dipita; Guha, Gunjan (2022-10-01). "Hormetic alteration of mTOR–mitochondria association: An approach to mitigate cellular aging". Current Opinion in Environmental Science & Health. 29: 100387. doi:10.1016/j.coesh.2022.100387. ISSN 2468-5844.
- ^ a b c Calabrese EJ (2014). "Hormesis: a fundamental concept in biology". Microbial Cell. 1 (5): 145–9. doi:10.15698/mic2014.05.145. PMC 5354598. PMID 28357236.
- ^ a b Hochberg CH, Semler MW, Brower RG (September 2021). "Oxygen toxicity in critically ill adults". American Journal of Respiratory and Critical Care Medicine. 204 (6): 632–641. doi:10.1164/rccm.202102-0417CI. PMC 8521700. PMID 34086536.
- ^ a b c Mattson, M. P (2007). "Hormesis defined". Ageing Research Reviews. 7 (1): 1–7. doi:10.1016/j.arr.2007.08.007. PMC 2248601. PMID 18162444.
- ^ Hayes, D. P. (2007). "Nutritional hormesis". European Journal of Clinical Nutrition. 61 (2): 147–159. doi:10.1038/sj.ejcn.1602507. ISSN 1476-5640. PMID 16885926.
- ^ a b c Kaiser, Jocelyn (2003). "Sipping from a Poisoned Chalice". Science. 302 (5644): 376–9. doi:10.1126/science.302.5644.376. PMID 14563981. S2CID 58523840.
- ^ Calabrese, Edward J. (2004). "Hormesis: A revolution in toxicology, risk assessment and medicine". EMBO Reports. 5 (Suppl 1): S37–40. doi:10.1038/sj.embor.7400222. PMC 1299203. PMID 15459733.
- ^ Bethell, Tom (2005). The Politically Incorrect Guide to Science. USA: Regnery Publishing. pp. 58–61. ISBN 978-0-89526-031-4.
- ^ Calabrese EJ, Baldwin LA (2001). "The frequency of U-shaped dose responses in the toxicological literature". Toxicological Sciences. 62 (2): 330–8. doi:10.1093/toxsci/62.2.330. PMID 11452146.
- ^ Hopper, Christopher P.; De La Cruz, Ladie Kimberly; Lyles, Kristin V.; Wareham, Lauren K.; Gilbert, Jack A.; Eichenbaum, Zehava; Magierowski, Marcin; Poole, Robert K.; Wollborn, Jakob; Wang, Binghe (2020-12-23). "Role of Carbon Monoxide in Host–Gut Microbiome Communication". Chemical Reviews. 120 (24): 13273–13311. doi:10.1021/acs.chemrev.0c00586. ISSN 0009-2665. PMID 33089988. S2CID 224824871.
- ^ Motterlini, Roberto; Otterbein, Leo E. (2010). "The therapeutic potential of carbon monoxide". Nature Reviews Drug Discovery. 9 (9): 728–743. doi:10.1038/nrd3228. ISSN 1474-1784. PMID 20811383. S2CID 205477130.
- ^ Hopper, Christopher P.; Zambrana, Paige N.; Goebel, Ulrich; Wollborn, Jakob (June 2021). "A brief history of carbon monoxide and its therapeutic origins". Nitric Oxide. 111–112: 45–63. doi:10.1016/j.niox.2021.04.001. PMID 33838343. S2CID 233205099.
- ^ Radak, Zsolt; Chung, Hae Y.; Koltai, Erika; Taylor, Albert W.; Goto, Sataro (2008). "Exercise, oxidative stress and hormesis". Ageing Research Reviews. 7 (1): 34–42. doi:10.1016/j.arr.2007.04.004. PMID 17869589. S2CID 20964603.
- ^ Bárcena, Clea; Mayoral, Pablo; Quirós, Pedro M. (1 January 2018). "Chapter Two - Mitohormesis, an Antiaging Paradigm" (Book series). In López-Otín, Carlos; Galluzzi, Lorenzo (eds.). International Review of Cell and Molecular Biology: Mitochondria and Longevity. Elsevier. pp. 35–77. ISBN 9780128157367. Retrieved 11 October 2021.
- ^ Sanz, Alberto; Stefanatos, Rhoda K.A. (1 March 2008). "The Mitochondrial Free Radical Theory of Aging: A Critical View". Current Aging Science. 1 (1): 10–21. doi:10.2174/1874609810801010010. PMID 20021368.
- ^ Brewer, Gregory J. (March 2010). "Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories". Experimental Gerontology. 45 (3): 173–179. doi:10.1016/j.exger.2009.11.007. PMC 2826600. PMID 19945522.
- ^ Bjelakovic, Goran; Nikolova, Dimitrinka; Gluud, Lise Lotte; Simonetti, Rosa G.; Gluud, Christian (28 February 2007). "Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention: Systematic Review and Meta-analysis". JAMA. 297 (8): 842–857. doi:10.1001/jama.297.8.842. PMID 17327526. Retrieved 11 October 2021.
- ^ Calabrese, Edward J.; Cook, Ralph (2006). "The Importance of Hormesis to Public Health". Environmental Health Perspectives. 114 (11): 1631–5. doi:10.1289/ehp.8606. JSTOR 4091789. PMC 1665397. PMID 17107845.
- ^ Fillmore, Kaye Middleton; Kerr, William C.; Stockwell, Tim; Chikritzhs, Tanya; Bostrom, Alan (2006). "Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies". Addiction Research & Theory. 14 (2): 101–32. doi:10.1080/16066350500497983. S2CID 72709357.
- ^ Fillmore, Kaye Middleton; Stockwell, Tim; Chikritzhs, Tanya; Bostrom, Alan; Kerr, William (2007). "Moderate Alcohol Use and Reduced Mortality Risk: Systematic Error in Prospective Studies and New Hypotheses". Annals of Epidemiology. 17 (5): S16–23. doi:10.1016/j.annepidem.2007.01.005. PMID 17478320.
- ^ Painter, Kelly; Cordell, Barbara J.; Sticco, Kristin L. (2021), "Auto-brewery Syndrome", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30020718, retrieved 2021-05-04
- ^ Yong, Ed (2019-09-20). "The Real Danger of Booze-Making Gut Bacteria". The Atlantic. Retrieved 2021-05-04.
- ^ Castro, Paola V.; Khare, Shilpi; Young, Brian D.; Clarke, Steven G. (2012). Singh, Shree Ram (ed.). "Caenorhabditis elegans Battling Starvation Stress: Low Levels of Ethanol Prolong Lifespan in L1 Larvae". PLOS ONE. 7 (1): e29984. Bibcode:2012PLoSO...729984C. doi:10.1371/journal.pone.0029984. PMC 3261173. PMID 22279556.
- ^ a b Heinz, Gary H.; Hoffman, David J.; Klimstra, Jon D.; Stebbins, Katherine R. (2010). "Enhanced reproduction in mallards fed a low level of methylmercury: An apparent case of hormesis". Environmental Toxicology and Chemistry. 29 (3): 650–3. Bibcode:2010EnvTC..29..650H. doi:10.1002/etc.64. PMID 20821490. S2CID 34149560.
- ^ Sutou, S. (2018). Low-dose radiation from A-bombs elongated lifespan and reduced cancer mortality relative to un-irradiated individuals. Genes and Environment, 40(1), 26. https://doi.org/10.1186/s41021-018-0114-3
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Sutou, Shizuyo (1 January 2020). "Black rain in Hiroshima: a critique to the Life Span Study of A-bomb survivors, basis of the linear no-threshold model". Genes and Environment. 42 (1): 1. Bibcode:2020GeneE..42....1S. doi:10.1186/s41021-019-0141-8. ISSN 1880-7062. PMC 6937943. PMID 31908690.
- ^ Sanders, Charles (2010). Sanders, Charles L. (ed.). Radiation Hormesis and the Linear-No-Threshold Assumption. Berlin: Springer. Bibcode:2010rhln.book.....S. doi:10.1007/978-3-642-03720-7. ISBN 978-3-642-42566-0.
- ^ "Ionizing radiation, health effects and protective measures". World Health Organization. Retrieved 2017-02-16.
- ^ Mothersill C, Seymour C (2009). "Implications for environmental health of multiple stressors". Journal of Radiological Protection. 29 (2A): A21–8. Bibcode:2009JRP....29...21M. doi:10.1088/0952-4746/29/2A/S02. PMID 19454807. S2CID 32270666.
- ^ a b c Latimer JJ, Alhamed A, Sveiven S, Almutairy A, Klimas NG, Abreu M, Sullivan K, Grant SG. Preliminary Evidence for a Hormetic Effect on DNA Nucleotide Excision Repair in Veterans with Gulf War Illness. Mil Med. 2020 Feb 13; 185(1–2):e47–e52. doi:10.1093/milmed/usz177. PMID 31334811; PMC PMC7353836.
- ^ Le Bourg, Eric; Rattan, Suresh, eds. (2008). Mild Stress and Healthy Aging: Applying hormesis in aging research and interventions. Springer. ISBN 978-1-4020-6868-3.[page needed]
- ^ Rattan, S. I. (2008). "Principles and practice of hormetic treatment of aging and age-related diseases". Human & Experimental Toxicology. 27 (2): 151–4. Bibcode:2008HETox..27..151R. doi:10.1177/0960327107083409. PMID 18480141. S2CID 504736.
- ^ a b c Rattan, Suresh I.S. (2008). "Hormesis in aging". Ageing Research Reviews. 7 (1): 63–78. doi:10.1016/j.arr.2007.03.002. PMID 17964227. S2CID 29221523.
- ^ a b Gems, David; Partridge, Linda (2008). "Stress-Response Hormesis and Aging: "That which Does Not Kill Us Makes Us Stronger"". Cell Metabolism. 7 (3): 200–3. doi:10.1016/j.cmet.2008.01.001. PMID 18316025.
- ^ Le Bourg; Rattan, eds. (2008). Mild Stress and Healthy Aging: Applying hormesis in aging research and interventions. Springer. ISBN 978-1-4020-6868-3.[page needed]
- ^ "Scientist says some pollution is good for you — a disputed claim Trump's EPA has embraced". Los Angeles Times. 2019-02-19. Retrieved 2020-08-11.
- ^ Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council (2005). Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. National Academies Press. ISBN 978-0-309-09156-5.[page needed]
- ^ a b Evaluation of the Linear-Nonthreshold Dose-Response Model for Ionizing Radiation. National Council on Radiation Protection and Measurements. 2001. ISBN 978-0-929600-69-7.[page needed]
- ^ Tubiana, Maurice (2005). "Dose–effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: The joint report of the Académie des Sciences (Paris) and of the Académie Nationale de Médecine". International Journal of Radiation Oncology, Biology, Physics. 63 (2): 317–9. doi:10.1016/j.ijrobp.2005.06.013. PMID 16168825.
- ^ Poumadere, M. (2003). Hormesis: public health policy, organizational safety and risk communication. Human & experimental toxicology, 22(1), 39-41
