Transfer-messenger RNA
| transfer-messenger RNA | |
|---|---|
 | |
| Identifiers | |
| Symbol | tmRNA |
| Rfam | RF00023 |
| Other data | |
| RNA type | gene |
| PDB structures | PDBe |
Transfer-messenger RNA (abbreviated tmRNA, also known as 10Sa RNA and by its genetic name SsrA) is a bacterial RNA molecule with dual tRNA-like and messenger RNA-like properties. The tmRNA forms a ribonucleoprotein complex (tmRNP) together with Small Protein B (SmpB), Elongation Factor Tu (EF-Tu), and ribosomal protein S1. In trans-translation, tmRNA and its associated proteins bind to bacterial ribosomes which have stalled in the middle of protein biosynthesis, for example when reaching the end of a messenger RNA which has lost its stop codon. The tmRNA is remarkably versatile: it recycles the stalled ribosome, adds a proteolysis-inducing tag to the unfinished polypeptide, and facilitates the degradation of the aberrant messenger RNA.[1] In the majority of bacteria these functions are carried out by standard one-piece tmRNAs. In other bacterial species, a permuted ssrA gene produces a two-piece tmRNA in which two separate RNA chains are joined by base-pairing.

Discovery and early work
[edit]tmRNA was first designated 10Sa RNA in 1979, after a mixed "10S" electrophoretic fraction of Escherichia coli RNA was further resolved into tmRNA and the similarly sized RNase P RNA (10Sb).[2] The presence of pseudouridine in the mixed 10S RNA hinted that tmRNA has modified bases found also in tRNA. The similarity at the 3' end of tmRNA to the T stem-loop of tRNA was first recognized upon sequencing ssrA from Mycobacterium tuberculosis.[3] Subsequent sequence comparison revealed the full tRNA-like domain (TLD) formed by the 5' and 3' ends of tmRNA, including the acceptor stem with elements like those in alanine tRNA that promote its aminoacylation by alanine-tRNA ligase.[4] It also revealed differences from tRNA: the anticodon arm is missing in tmRNA, and the D arm region is a loop without base pairs.
Structure
[edit]Secondary structure of the standard one-piece tmRNAs
[edit]
The complete E. coli tmRNA secondary structure was elucidated by comparative sequence analysis and structural probing.[5][6] Watson-Crick and G-U base pairs were identified by comparing the bacterial tmRNA sequences using automated computational methods in combination with manual alignment procedures.[7][8] The accompanying figure shows the base pairing pattern of this prototypical tmRNA, which is organized into 12 phylogenetically supported helices (also called pairings P1 to P12), some divided into helical segments.
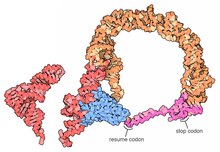
A prominent feature of every tmRNA is the conserved tRNA-like domain (TLD), composed of helices 1, 12, and 2a (analogs of the tRNA acceptor stem, T-stem and variable stem, respectively), and containing the 5' monophosphate and alanylatable 3' CCA ends. The mRNA-like region (MLR) is in standard tmRNA a large loop containing pseudoknots and a coding sequence (CDS) for the tag peptide, marked by the resume codon and the stop codon. The encoded tag peptide (ANDENYALAA in E. coli) varies among bacteria, perhaps depending on the set of proteases and adaptors available.[9]
tmRNAs typically contain four pseudoknots, one (pk1) upstream of the tag peptide CDS, and the other three pseudoknots (pk2 to pk4) downstream of the CDS. The pseudoknot regions, although generally conserved, are evolutionarily plastic. For example, in the (one-piece) tmRNAs of cyanobacteria, pk4 is substituted with two tandemly arranged smaller pseudoknots. This suggests that tmRNA folding outside the TLD can be important, yet the pseudoknot region lacks conserved residues and pseudoknots are among the first structures to be lost as ssrA sequences diverge in plastid and endosymbiont lineages. Base pairing in the three-pseudoknot region of E. coli tmRNA is disrupted during trans-translation.[7][10]
Two-piece tmRNAs
[edit]Circularly permuted ssrA has been reported in three major lineages: i) all alphaproteobacteria and the primitive mitochondria of jakobid protists, ii) two disjoint groups of cyanobacteria (Gloeobacter and a clade containing Prochlorococcus and many Synechococcus), and iii) some members of the betaproteobacteria (Cupriavidus and some Rhodocyclales).[11][12] All produce the same overall two-piece (acceptor and coding pieces) form, equivalent to the standard form nicked downstream of the reading frame. None retain more than two pseudoknots compared to the four (or more) of standard tmRNA.
Alphaproteobacteria have two signature sequences: replacement of the typical T-loop sequence TΨCRANY with GGCRGUA, and the sequence AACAGAA in the large loop of the 3´-terminal pseudoknot. In mitochondria, the MLR has been lost, and a remarkable re-permutation of mitochondrial ssrA results in a small one-piece product in Jakoba libera.[13]
The cyanobacteria provide the most plausible case for evolution of a permuted gene from a standard gene, due to remarkable sequence similarities between the two gene types as they occur in different Synechococcus strains.
tmRNA processing
[edit]Most tmRNAs are transcribed as larger precursors which are processed much like tRNA. Cleavage at the 5´ end is by ribonuclease P.[4] Multiple exonucleases can participate in the processing of the 3´ end of tmRNA, although RNase T and RNase PH are most effective.[14][15] Depending on the bacterial species, the 3'-CCA is either encoded or added by tRNA nucleotidyltransferase.
Similar processing at internal sites of permuted precursor tmRNA explains its physical splitting into two pieces. The two-piece tmRNAs have two additional ends whose processing must be considered. For alphaproteobacteria, one 5´ end is the unprocessed start site of transcription.[16] The far 3´ end may in some cases be the result of rho-independent termination.
Three-dimensional structures
[edit]

High-resolution structures of the complete tmRNA molecules are currently unavailable and may be difficult to obtain due to the inherent flexibility of the MLR. In 2007, the crystal structure of the Thermus thermophilus TLD bound to the SmpB protein was obtained at 3 Å resolution. This structure shows that SmpB mimics the D stem and the anticodon of a canonical tRNA whereas helical section 2a of tmRNA corresponds to the variable arm of tRNA.[18] A cryo-electron microscopy study of tmRNA at an early stage of trans-translation shows the spatial relationship between the ribosome and the tmRNP (tmRNA bound to the EF-Tu protein). The TLD is located near the GTPase-associated center in the 50S ribosomal subunit; helix 5 and pseudoknots pk2 to pk4 form an arc around the beak of the 30S ribosomal subunit.[19]
Trans-translation
[edit]
Coding by tmRNA was discovered in 1995[20] when Simpson and coworkers overexpressed the mouse cytokine IL-6 in E. coli and found multiple truncated cytokine-derived peptides each tagged at the carboxyl termini with the same 11-amino acid residue extension (A)ANDENYALAA. With the exception of the N-terminal alanine, which comes from the 3' end of tmRNA itself, this tag sequence was traced to a short open reading frame in E. coli tmRNA. Keiler, et al., recognized that the tag peptide confers proteolysis and proposed the trans-translation model for tmRNA action.[21]
While details of the trans-translation mechanism are under investigation it is generally agreed that tmRNA first occupies the empty A site of the stalled ribosome. Subsequently, the ribosome moves from the 3' end of the truncated messenger RNA onto the resume codon of the MLR, followed by a slippage-prone stage from where translation continues normally until the in-frame tmRNA stop codon is encountered. Trans-translation is essential in some bacterial species, whereas other bacteria require tmRNA to survive when subjected to stressful growth conditions.[22] It is believed that tmRNA can help the cell with antibiotic resistance by rescuing the ribosomes stalled by antibiotics.[23] Depending on the organism, the tag peptide may be recognized by a variety of proteases or protease adapters.[9]
Mobile genetic elements and the tmRNA gene
[edit]
ssrA is both a target for some mobile DNAs and a passenger on others. It has been found interrupted by three types of mobile elements. By different strategies none of these disrupt gene function: group I introns remove themselves by self-splicing, rickettsial palindromic elements (RPEs) insert in innocuous sites, and integrase-encoding genomic islands split their target ssrA yet restore the split-off portion.[24][25][26][27]
Non-chromosomal ssrA was first detected in a genomic survey of mycobacteriophages (in 10% of the phages).[28] Other mobile elements including plasmids and genomic islands have been found bearing ssrA. One interesting case is Rhodobacter sphaeroides ATCC 17025, whose native tmRNA gene is disrupted by a genomic island; unlike all other genomic islands in tmRNA (or tRNA) genes this island has inactivated the native target gene without restoration, yet compensates by carrying its own tmRNA gene. A very unusual relative of ssrA is found in the lytic mycobacteriophage DS6A, that encodes little more than the TLD.
Mitochondrial tmRNAs (ssrA gene)
[edit]A mitochondrion-encoded, structurally reduced form of tmRNA (mt-tmRNA) was first postulated for the jakobid flagellate Reclinomonas americana.[11] Subsequently, the presence of a mitochondrial gene (ssrA) coding for tmRNA, as well as transcription and RNA processing sites were confirmed for all but one member of jakobids.[29][13] Functional evidence, i.e., mt-tmRNA Aminoacylation with alanine, is available for Jakoba libera.[13] More recently, ssrA was also identified in mitochondrial genomes of oomycetes.[30] Like in α-Proteobacteria (the ancestors of mitochondria), mt-tmRNAs are circularly permuted, two-piece RNA molecules, except in Jakoba libera where the gene has reverted to encoding a one-piece tmRNA conformation.[13]
Identification of ssrA in mitochondrial genomes
[edit]
Mitochondrial tmRNA genes were initially recognized as short sequences that are conserved among jakobids and that have the potential to fold into a distinct tRNA-like secondary structure. With the availability of nine complete jakobid mtDNA sequences,[29] and a significantly improved covariance search tool (Infernal;[31][32][33]), a covariance model has been developed based on jakobid mitochondrial tmRNAs, which identified mitochondrial ssrA genes also in oomycete. At present, a total of 34 oomycete mt-tmRNAs have been detected across six genera: Albugo, Bremia, Phytophthora, Pseudoperonospora, Pythium and Saprolegnia. A covariance model built with both jakobid and oomycete sequences is now available at Rfam under the name ‘mt-tmRNA’.[30]
mt-tmRNA Structure
[edit]The standard bacterial tmRNA consists of a tRNA(Ala)-like domain (allowing addition of a non-encoded alanine to mRNAs that happen to lack a stop coding), and an mRNA-like domain coding for a protein tag that destines the polypeptide for proteolysis. The mRNA-like domain was lost in mt-tmRNAs. Comparative sequence analysis indicates features typical for mt-tmRNAs.[30] Most conserved is the primary sequence of the amino acyl acceptor stem. This portion of the molecule has an invariable A residue in the discriminator position and a G-U pair at position 3 (except in Seculamonas ecuadoriensis, which has a G-C pair); this position is the recognition site for alanyl tRNA synthase. P2 is a helix of variable length (3 to 10 base pairs) and corresponds to the anticodon stem of tRNAs, yet without an anticodon loop (as not required for tmRNA function). P2 stabilizes the tRNA-like structure, but four nucleotides invariant across oomycetes and jakobids suggest an additional, currently unidentified function. P3 has five base pairs and corresponds to the T-arm of tRNAs, yet with different consensus nucleotides both in the paired region and the loop. The T-loop sequence is conserved across oomycetes and jakobid, with only few deviations (e.g., Saprolegnia ferax). Finally, instead of the tRNA-like D-stem with a shortened three-nucleotide D-loop characteristic for bacterial tmRNAs, mitochondrial counterparts have a highly variable 5 to 14-nt long loop. The intervening sequence (Int.) of two-piece mt-tmRNAs is A+U rich and of irregular length (4-34 nt). ). For secondary structure models of one- and two-piece mt-tmRNAs see Figure 1.
mt-tmRNA processing and expression
[edit]
RNA-Seq data of Phytophthora sojae show an expression level similar to that of neighboring mitochondrial tRNAs, and four major processing sites confirm the predicted termini of mature mt-tmRNA.[30] The tmRNA precursor molecule is likely processed by RNase P and a tRNA 3’ processing endonuclease (see Figure 2); the latter activity is assumed to lead to the removal of the intervening sequence. Following the addition of CCA at the 3’ discriminator nucleotide, the tmRNA can be charged by alanyl-tRNA synthetase with alanine.
See also
[edit]References
[edit]- ^ Keiler KC (2008). "Biology of trans-translation". Annual Review of Microbiology. 62: 133–51. doi:10.1146/annurev.micro.62.081307.162948. PMID 18557701.
- ^ Ray BK, Apirion D (July 1979). "Characterization of 10S RNA: a new stable rna molecule from Escherichia coli". Molecular & General Genetics. 174 (1): 25–32. doi:10.1007/BF00433301. PMID 384159. S2CID 22699560.
- ^ Tyagi JS, Kinger AK (January 1992). "Identification of the 10Sa RNA structural gene of Mycobacterium tuberculosis". Nucleic Acids Research. 20 (1): 138. doi:10.1093/nar/20.1.138. PMC 310338. PMID 1371186.
- ^ a b Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H (September 1994). "A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America. 91 (20): 9223–7. Bibcode:1994PNAS...91.9223K. doi:10.1073/pnas.91.20.9223. PMC 44784. PMID 7524073.
- ^ Williams KP, Bartel DP (December 1996). "Phylogenetic analysis of tmRNA secondary structure". RNA. 2 (12): 1306–10. PMC 1369456. PMID 8972778.
- ^ Felden B, Himeno H, Muto A, McCutcheon JP, Atkins JF, Gesteland RF (January 1997). "Probing the structure of the Escherichia coli 10Sa RNA (tmRNA)". RNA. 3 (1): 89–103. PMC 1369465. PMID 8990402.
- ^ a b Zwieb C, Wower I, Wower J (May 1999). "Comparative sequence analysis of tmRNA". Nucleic Acids Research. 27 (10): 2063–71. doi:10.1093/nar/27.10.2063. PMC 148424. PMID 10219077.
- ^ Andersen ES, Lind-Thomsen A, Knudsen B, Kristensen SE, Havgaard JH, Torarinsson E, Larsen N, Zwieb C, Sestoft P, Kjems J, Gorodkin J (November 2007). "Semiautomated improvement of RNA alignments". RNA. 13 (11): 1850–9. doi:10.1261/rna.215407. PMC 2040093. PMID 17804647.
- ^ a b Gur E, Sauer RT (October 2008). "Evolution of the ssrA degradation tag in Mycoplasma: specificity switch to a different protease". Proceedings of the National Academy of Sciences of the United States of America. 105 (42): 16113–8. Bibcode:2008PNAS..10516113G. doi:10.1073/pnas.0808802105. PMC 2570983. PMID 18852454.
- ^ Wower IK, Zwieb C, Wower J (May 2005). "Transfer-messenger RNA unfolds as it transits the ribosome". RNA. 11 (5): 668–73. doi:10.1261/rna.7269305. PMC 1370753. PMID 15811920.
- ^ a b Keiler KC, Shapiro L, Williams KP (July 2000). "tmRNAs that encode proteolysis-inducing tags are found in all known bacterial genomes: A two-piece tmRNA functions in Caulobacter". Proceedings of the National Academy of Sciences of the United States of America. 97 (14): 7778–83. Bibcode:2000PNAS...97.7778K. doi:10.1073/pnas.97.14.7778. PMC 16621. PMID 10884408.
- ^ Sharkady SM, Williams KP (2004). "A third lineage with two-piece tmRNA". Nucleic Acids Research. 32 (15): 4531–8. doi:10.1093/nar/gkh795. PMC 516066. PMID 15326226.
- ^ a b c d Jacob Y, Seif E, Paquet PO, Lang BF (April 2004). "Loss of the mRNA-like region in mitochondrial tmRNAs of jakobids". RNA. 10 (4): 605–14. doi:10.1261/rna.5227904. PMC 1370551. PMID 15037770.
- ^ Srivastava RA, Srivastava N, Apirion D (May 1992). "Characterization of the RNA processing enzyme RNase III from wild type and overexpressing Escherichia coli cells in processing natural RNA substrates". The International Journal of Biochemistry. 24 (5): 737–49. doi:10.1016/0020-711X(92)90007-N. PMID 1375563.
- ^ Li Z, Pandit S, Deutscher MP (March 1998). "3' exoribonucleolytic trimming is a common feature of the maturation of small, stable RNAs in Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America. 95 (6): 2856–61. Bibcode:1998PNAS...95.2856L. doi:10.1073/pnas.95.6.2856. PMC 19659. PMID 9501180.
- ^ Mao C, Bhardwaj K, Sharkady SM, Fish RI, Driscoll T, Wower J, Zwieb C, Sobral BW, Williams KP (2009). "Variations on the tmRNA gene". RNA Biology. 6 (4): 355–61. doi:10.4161/rna.6.4.9172. PMID 19617710.
- ^ Someya T, Nameki N, Hosoi H, Suzuki S, Hatanaka H, Fujii M, Terada T, Shirouzu M, Inoue Y, Shibata T, Kuramitsu S, Yokoyama S, Kawai G (January 2003). "Solution structure of a tmRNA-binding protein, SmpB, from Thermus thermophilus". FEBS Letters. 535 (1–3): 94–100. Bibcode:2003FEBSL.535...94S. doi:10.1016/S0014-5793(02)03880-2. PMID 12560085.
- ^ a b Bessho Y, Shibata R, Sekine S, Murayama K, Higashijima K, Hori-Takemoto C, Shirouzu M, Kuramitsu S, Yokoyama S (May 2007). "Structural basis for functional mimicry of long-variable-arm tRNA by transfer-messenger RNA". Proceedings of the National Academy of Sciences of the United States of America. 104 (20): 8293–8. Bibcode:2007PNAS..104.8293B. doi:10.1073/pnas.0700402104. PMC 1895943. PMID 17488812.
- ^ Valle M, Gillet R, Kaur S, Henne A, Ramakrishnan V, Frank J (April 2003). "Visualizing tmRNA entry into a stalled ribosome". Science. 300 (5616): 127–30. Bibcode:2003Sci...300..127V. doi:10.1126/science.1081798. PMID 12677067. S2CID 28845151.
- ^ Tu GF, Reid GE, Zhang JG, Moritz RL, Simpson RJ (April 1995). "C-terminal extension of truncated recombinant proteins in Escherichia coli with a 10Sa RNA decapeptide". The Journal of Biological Chemistry. 270 (16): 9322–6. doi:10.1074/jbc.270.16.9322. PMID 7536743.
- ^ Keiler KC, Waller PR, Sauer RT (February 1996). "Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA". Science. 271 (5251): 990–3. Bibcode:1996Sci...271..990K. doi:10.1126/science.271.5251.990. PMID 8584937. S2CID 29254050.
- ^ Thibonnier M, Thiberge JM, De Reuse H (2008). Ahmed N (ed.). "Trans-translation in Helicobacter pylori: essentiality of ribosome rescue and requirement of protein tagging for stress resistance and competence". PLOS ONE. 3 (11): e3810. Bibcode:2008PLoSO...3.3810T. doi:10.1371/journal.pone.0003810. PMC 2584231. PMID 19043582.
- ^ Müller, Claudia; Crowe-McAuliffe, Caillan; Wilson, Daniel N. (2021-03-18). "Ribosome Rescue Pathways in Bacteria". Frontiers in Microbiology. 12: 652980. doi:10.3389/fmicb.2021.652980. ISSN 1664-302X. PMC 8012679. PMID 33815344.
- ^ Kirby JE, Trempy JE, Gottesman S (April 1994). "Excision of a P4-like cryptic prophage leads to Alp protease expression in Escherichia coli". Journal of Bacteriology. 176 (7): 2068–81. doi:10.1128/jb.176.7.2068-2081.1994. PMC 205313. PMID 7511583.
- ^ Williams KP (January 2002). "The tmRNA Website: invasion by an intron". Nucleic Acids Research. 30 (1): 179–82. doi:10.1093/nar/30.1.179. PMC 99078. PMID 11752287.
- ^ Dwyer DS (January 2001). "Selfish DNA and the origin of genes". Science. 291 (5502): 252–3. doi:10.1126/science.291.5502.252. PMID 11253208. S2CID 5369275.
- ^ Williams KP (February 2003). "Traffic at the tmRNA gene". Journal of Bacteriology. 185 (3): 1059–70. doi:10.1128/JB.185.3.1059-1070.2003. PMC 142792. PMID 12533482.
- ^ Hatfull GF, Pedulla ML, Jacobs-Sera D, Cichon PM, Foley A, Ford ME, Gonda RM, Houtz JM, Hryckowian AJ, Kelchner VA, Namburi S, Pajcini KV, Popovich MG, Schleicher DT, Simanek BZ, Smith AL, Zdanowicz GM, Kumar V, Peebles CL, Jacobs WR, Lawrence JG, Hendrix RW (June 2006). "Exploring the mycobacteriophage metaproteome: phage genomics as an educational platform". PLOS Genetics. 2 (6): e92. doi:10.1371/journal.pgen.0020092. PMC 1475703. PMID 16789831.
- ^ a b Burger G, Gray MW, Forget L, Lang BF (2013). "Strikingly bacteria-like and gene-rich mitochondrial genomes throughout jakobid protists". Genome Biology and Evolution. 5 (2): 418–38. doi:10.1093/gbe/evt008. PMC 3590771. PMID 23335123.
- ^ a b c d Hafez M, Burger G, Steinberg SV, Lang BF (July 2013). "A second eukaryotic group with mitochondrion-encoded tmRNA: in silico identification and experimental confirmation". RNA Biology. 10 (7): 1117–24. doi:10.4161/rna.25376. PMC 3849159. PMID 23823571. Archived from the original on 2014-02-21. Retrieved 2014-02-13.
- ^ Eddy, S. "Infernal website". Retrieved 14 August 2016.
- ^ Eddy SR, Durbin R (June 1994). "RNA sequence analysis using covariance models". Nucleic Acids Research. 22 (11): 2079–88. doi:10.1093/nar/22.11.2079. PMC 308124. PMID 8029015.
- ^ Nawrocki EP, Kolbe DL, Eddy SR (May 2009). "Infernal 1.0: inference of RNA alignments". Bioinformatics. 25 (10): 1335–7. doi:10.1093/bioinformatics/btp157. PMC 2732312. PMID 19307242.
Further reading
[edit]- Hong SJ, Tran QA, Keiler KC (July 2005). "Cell cycle-regulated degradation of tmRNA is controlled by RNase R and SmpB". Molecular Microbiology. 57 (2): 565–75. doi:10.1111/j.1365-2958.2005.04709.x. PMC 3776457. PMID 15978085.
